Abstract
Background Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired life-threatening disease characterized by complement-mediated hemolysis and thrombosis. Chronic hemolysis leads to anemia, which can result in persistent fatigue and dependance on transfusions, affecting the overall quality of life (QOL) of patients with PNH. Pegcetacoplan (PEG; US brand name Empaveli®) is the first approved C3 inhibitor for US adults with PNH. Although clinical trials have assessed the efficacy of PEG, which include patient-reported outcomes, there is no information on the use of PEG in a real-world setting.
Aims To provide the first report of trends in hemoglobin (Hb) values and baseline QOL (measured by patient-reported fatigue and cognitive impairment) from OPERA, a descriptive, observational exploratory outcomes study, presenting the first real-world data describing PEG treatment for adults with PNH post-approval.
Methods This single site opt-in study recruited patients in the US, following a valid PEG prescription from a licensed medical professional. Patients ≥18 years of age, diagnosed with PNH and prescribed PEG, were electronically consented and then enrolled to participate in the OPERA study, as approved by the internal review board. OPERA collected information from routine care and did not direct or suggest any medical interventions. In this observational setting, Hb lab values were reported (by the patient/site) at varying timepoints when available. Participants self-reported QOL status from baseline over a 12-month period through monthly phone calls and quarterly online patient-reported outcome measures (PROMs). During monthly phone calls, participants were asked to report their most recent Hb levels (typical normal range: male, 13.6-18 g/dL; female, 12-16 g/dL) if available from their regular primary care visits in the previous month. Online PROMs collected data using validated QOL instruments including the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scale (0-52 total score) and the Patient-Reported Outcomes Measurement Information System (PROMIS) scale for Cognitive Abilities (23.27-67.09 t-score). Lower scores on each measure represent greater levels of fatigue or cognitive impairment.
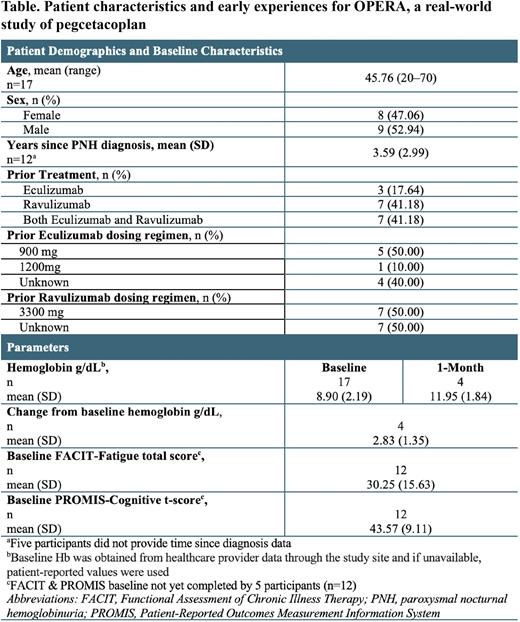
Results Participants enrolled in OPERA with a 30-70% monthly range to opt-in. Of 17 participants enrolled with available baseline Hb values, mean (range) age was 45.76 (20-70) years, and 8 were female. No OPERA participants were previously enrolled in a PEG clinical trial. All participants were prescribed and received the label recommended dose of PEG (1080 mg, twice weekly). All participants reported prior treatment with either eculizumab, ravulizumab or both, before starting PEG. Additional dosing information and time since PNH diagnosis can be found in the Table. The mean (SD) baseline Hb levels from 17 OPERA participants was 8.90 (2.19) g/dL, which was similar to the mean (SD) baseline Hb levels (8.69 [1.08] g/dL) for 41 patients in the previously published phase 3 PEGASUS (NCT03500549) trial. Among 4 participants with available data after 1 month of PEG use, mean (SD) Hb levels increased to 11.95 (1.84) g/dL (mean change from baseline +2.83 (1.35) g/dL) which was similar to the mean (SD) Hb levels of 11.55 (1.62) g/dL seen in 40 PEGASUS participants after 1 month of treatment. QOL data was available at baseline in 12 participants and the mean (SD) baseline FACIT-Fatigue score was 30.25 (15.63), which was similar to the mean (SD) baseline FACIT-Fatigue score of 32.2 (11.4) from the PEGASUS trial, but below the reported mean FACIT-Fatigue population norm of 43.6 (Cella, et al. Cancer. 2002). Additionally, the mean (SD) PROMIS-Cognitive Abilities t-score for patients with available QOL data (n=12) was 43.57 (9.11), which is lower than the published population norm of 50 (Saffer, et al. Psychiatry Research 2015).
Conclusions This first real-world study of adults with PNH receiving PEG indicates a positive trend in Hb levels in participants after 1 month of use. These early experiences are similar to previously reported changes in Hb levels after PEG treatment in phase 3 clinical trials (Hillmen et al, NEJM 2021). At baseline, participants with PNH report a considerable amount of fatigue and cognitive impairment, indicating poor QOL with previous C5 inhibitor treatment. More research is needed to corroborate the findings of this first analysis.
Disclosures
Fishman:Apellis Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Min:Apellis Pharmaceuticals: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal